Our laboratory is interested in the role of epigenetics in human autism-spectrum disorders. Epigenetics is the study of heritable changes in chromosomes that are not encoded in the DNA sequence, including DNA methylation and chromatin organization. The clinical applications of our research include understanding the pathogenesis of the neurodevelopmental disorders such Autism Spectrum Disorders (ASDs), Rett syndrome, Prader-Willi syndrome, and Angelman syndrome.
Research Areas
Genomic Imprinting: Our group has long been interested in genomic imprinting, which creates parent-of-origin specific gene expression profiles by making use of epigenetic marks. Genomic imprinting is a key component of neurodevelopment and is particularly environmentally responsive during certain developmental windows. Thus, an understanding of genomic imprinting is critical in understanding the origins of ASDs and how gene-by-environment interactions shape the brain. Towards this end, we have intensely investigated the Snrpn-Ube3a locus, where genetic mutations result in the sister disorders: Prader-Willi syndrome and Angelman syndrome. This region has captivated our interest as it produces proteins and non-coding RNAs that shape neurodevelopment, metabolism, and circadian rhythm.
Autism Spectrum Disorders: ASDs represent a large and diverse spectrum of neurodevelopmental disorders. In order to understand the molecular basis of ASDs, our group has focused in on ASDs with a known genetic cause. In these ASDs, a portion of chromosome 15, which contains Ube3a, has been duplicated (Dup15q). Dup15q is not only associated with ASDs but also with exposure to environmental pollutants and has deepened our understanding of how gene-by-environment interactions shape the risk for ASDs. We also examine ASDs with unknown causes, as they represent the vast majority of cases, and are working towards developing biomarkers for ASD diagnosis by using non-invasively obtained tissues such as placenta, cord blood, and buccal swabs.
MeCP2: We are also focused on a protein that binds to methylated DNA: methyl CpG binding protein 2 (MeCP2). The gene for MECP2 is on the X chromosome and is mutated in Rett syndrome and other neurodevelopmental disorders. We are currently testing the role of MeCP2 in the regulation of gene expression and the organization of parentally imprinted chromosomes. We are also investigating the role of MeCP2 in chromatin dynamics neuronal ontogeny during post-natal neuronal maturation in the cerebral cortex.
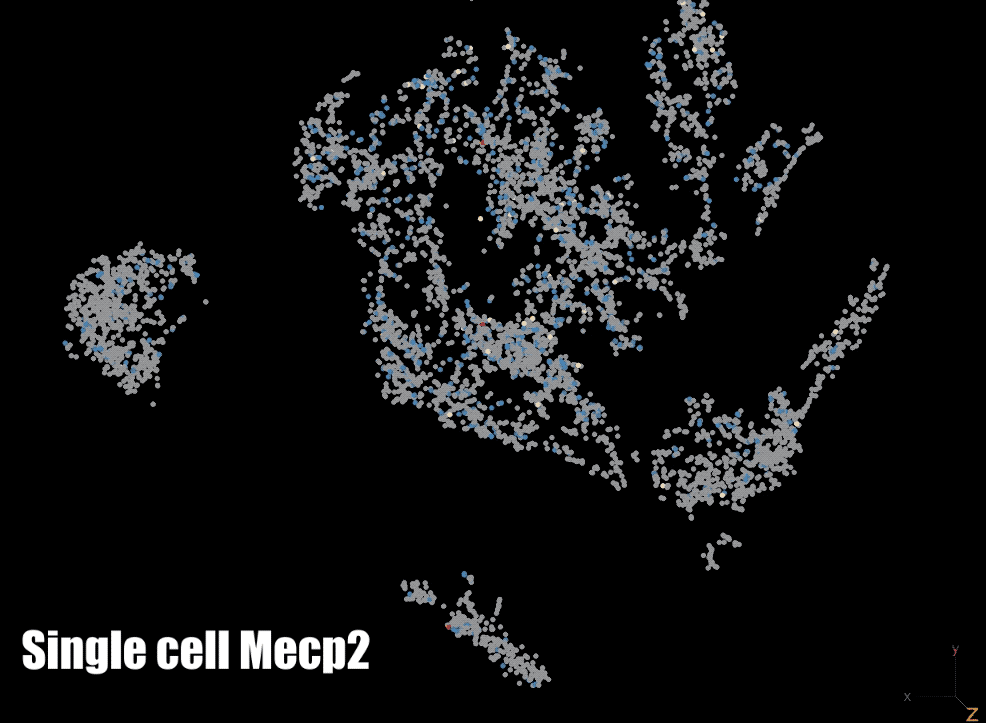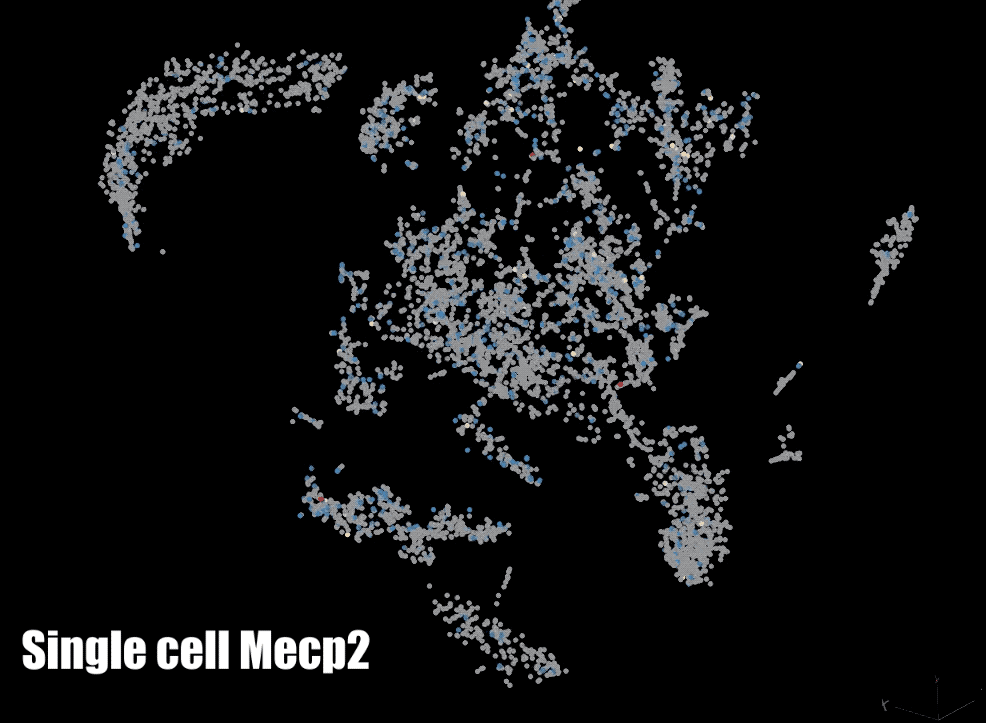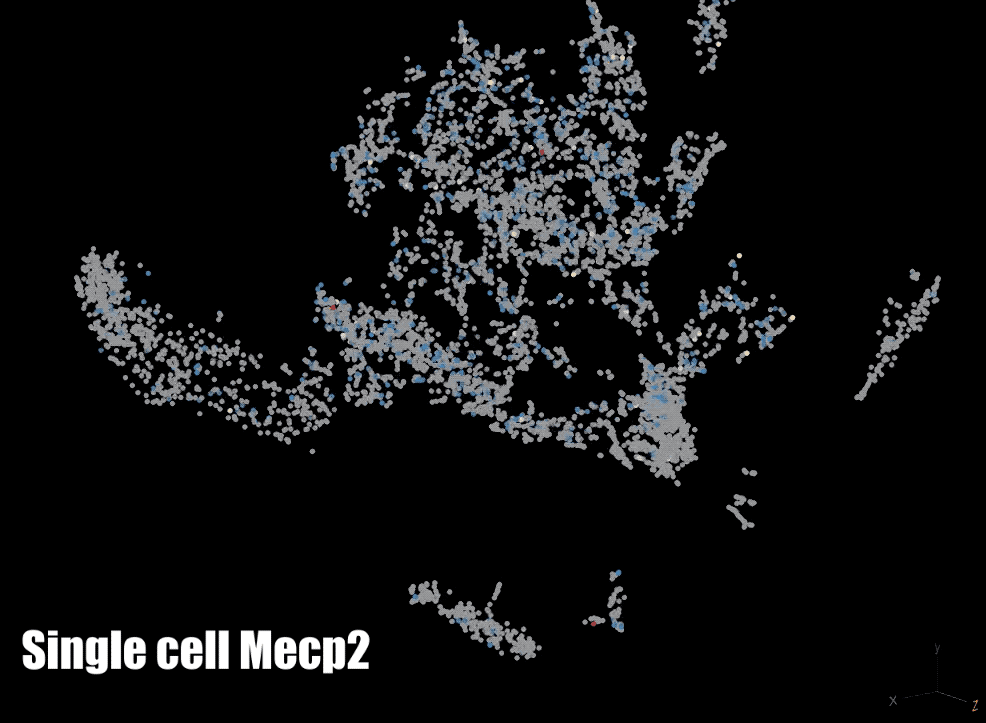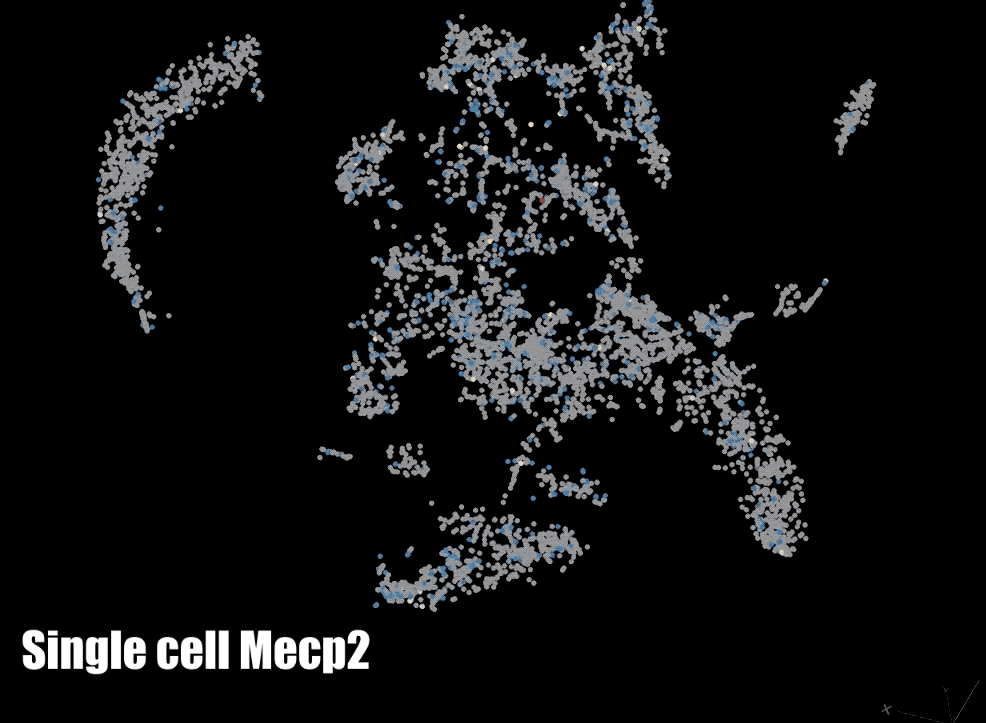
Biotechnology: In order to interrogate DNA methylation we make use of a number of technologies to examine it globally and/or at a gene specific level. We have developed a custom whole-genome bisulfite sequencing toolset that enables epidemiological level studies of genome-wide DNA methylation and we also utilize pyrosequencing to examine DNA methylation at specific regions. Finally, we also make use of a number of other methods such as single-cell RNA sequencing (scRNA-seq), fluorescent in situ hybridization (FISH), chromatin immunoprecipitation with sequencing (ChIP-seq), assay for transposase-accessible chromatin with sequencing (ATAC-seq), and reverse transcriptase quantitative polymerase chain reaction (RT-qPCR).




















































